07.08.2019
The chemistry of the elements is aluminum. What is aluminum
Section 1. Name and history of the discovery of aluminum.
Section 2. General Description aluminum, physical and chemical properties.
Section 3. Obtaining castings from aluminum alloys.
Section 4. Application aluminum.
Aluminum Is an element of the main subgroup of the third group, the third period of the periodic system of chemical elements of D. I. Mendeleev, with atomic number 13. It is designated by the symbol Al. It belongs to the group of light metals. Most common metal and the third most abundant chemical element in the earth's crust (after oxygen and silicon).
Simple substance aluminum (CAS number: 7429-90-5) - lightweight, paramagnetic metal silver-white color, easily moldable, cast, machined. Aluminum has high thermal and electrical conductivity, corrosion resistance due to the rapid formation of strong oxide films that protect the surface from further interaction.
The achievements of industry in any developed society are invariably associated with the achievements of the technology of structural materials and alloys. The quality of processing and the productivity of manufacturing items of trade are the most important indicators of the level of development of the state.
The materials used in modern designs, in addition to high strength characteristics, should have a set of properties such as increased corrosion resistance, heat resistance, heat conductivity and electrical conductivity, refractoriness, as well as the ability to maintain these properties under long-term operation under loads.
Scientific developments and production processes in the field of non-ferrous metal foundry in our country correspond to the advanced achievements of scientific and technological progress. Their result, in particular, was the creation of modern chill and injection molding workshops at the Volga Automobile Plant and a number of other enterprises. Large injection molding machines with a locking force of the mold 35 MN are successfully operating at the Zavolzhsky Motor Plant, which produce cylinder blocks of aluminum alloys for the Volga car.
At the Altai Motor Plant, an automated line for producing injection-molded castings has been mastered. In the Union of Soviet Socialist Republics (), the world's first developed and mastered process continuous casting of ingots of aluminum alloys in an electromagnetic mold. This method significantly improves the quality of the ingots and reduces the amount of waste in the form of chips during turning.
Title and history of the discovery of aluminum
Latin aluminum comes from the Latin alumen, meaning alum (aluminum and potassium sulfate (K) KAl (SO4) 2 · 12H2O), which have long been used in leather dressing and as an astringent. Al, a chemical element of group III of the periodic system, atomic number 13, atomic mass 26, 98154. Due to the high chemical activity, the discovery and release of pure aluminum lasted almost 100 years. The conclusion that alum can be obtained “” (a refractory substance, in modern day - aluminum oxide) made back in 1754. German chemist A. Markgraf. It later turned out that the same “earth” could be extracted from clay, and it was called alumina. He was able to get metallic aluminum only in 1825. Danish physicist H.K. Oersted. He treated with a potassium amalgam (potassium alloy (K) with mercury (Hg)) aluminum chloride AlCl3, which could be obtained from alumina, and after distillation of the mercury (Hg) he isolated gray aluminum powder.
Only after a quarter of a century this method was able to be slightly modernized. The French chemist A.E. St. Clair Deville in 1854 proposed using metallic sodium (Na) to produce aluminum, and received the first ingots of the new metal. The cost of aluminum was then very high, and jewelry was made from it.
An industrial method for producing aluminum by electrolysis of the melt of complex mixtures, including aluminum oxide, aluminum fluoride and other substances, was independently developed in 1886 by P. Eru () and C. Hall (USA). Aluminum production is associated with high electricity costs, so it was sold on a large scale only in the 20th century. IN Union of Soviet Socialist Republics (CCCP) The first industrial aluminum was obtained on May 14, 1932 at the Volkhov aluminum plant, built near the Volkhov hydroelectric power station.
Aluminum with a purity in excess of 99, 99% was first obtained by electrolysis in 1920. In 1925 in work Edwards published some information on the physical and mechanical properties of such aluminum. In 1938 Taylor, Wheeler, Smith and Edwards published an article that lists some properties of 99.96% pure aluminum, also obtained by electrolysis in France. The first edition of the monograph on the properties of aluminum was published in 1967.

In subsequent years, due to the comparative simplicity of preparation and attractive properties, many works about the properties of aluminum. Pure aluminum is widely used mainly in electronics - from electrolytic capacitors to the pinnacle of electronic engineering - microprocessors; in cryoelectronics, cryomagnetics.


Newer methods for producing pure aluminum are the zone cleaning method, crystallization from amalgams (aluminum alloys with mercury), and separation from alkaline solutions. The degree of purity of aluminum is controlled by the value of electrical resistance at low temperatures.
General characteristics of aluminum
Natural aluminum consists of one 27Al nuclide. The configuration of the external electron layer 3s2p1. In almost all compounds, the oxidation state of aluminum is +3 (valency III). The radius of the neutral atom of aluminum is 0, 143 nm, the radius of the ion is Al3 + 0, 057 nm. The sequential ionization energies of the neutral aluminum atom are 5, 984, 18, 828, 28, 44, and 120 eV, respectively. On the Pauling scale, the electronegativity of aluminum is 1, 5.

Aluminum - soft, light, silver-white, the crystal lattice of which is cubic face-centered, parameter a \u003d 0, 40403 nm. The melting point of pure metal is 660 ° C, the boiling point is about 2450 ° C, density 2, 6989 g / cm3. The temperature coefficient of linear expansion of aluminum is about 2.5 · 10-5 K-1.
Chemical aluminum is a fairly active metal. In air, its surface is instantly covered with a dense film of Al2O3 oxide, which prevents further access of oxygen (O) to the metal and leads to the termination of the reaction, which causes high anticorrosion properties of aluminum. A protective surface film on aluminum also forms if it is placed in concentrated nitric acid.
Aluminum actively reacts with other acids:
6CHl + 2Al \u003d 2AlCl3 + 3H2,
3H2SO4 + 2Al \u003d Al2 (SO4) 3 + 3H2.
Interestingly, the reaction between aluminum and iodine (I) powders begins at room temperature if a few drops of water are added to the initial mixture, which in this case plays the role of a catalyst:
2Al + 3I2 \u003d 2AlI3.
The interaction of aluminum with sulfur (S) when heated leads to the formation of aluminum sulfide:
2Al + 3S \u003d Al2S3,
which is easily decomposed by water:
Al2S3 + 6H2O \u003d 2Al (OH) 3 + 3H2S.
Aluminum does not directly interact with hydrogen (H), but indirectly, for example, using organoaluminum compounds, it is possible to synthesize solid polymer aluminum hydride (AlH3) x, the strongest reducing agent.
In the form of a powder, aluminum can be burned in air, and a white refractory powder of aluminum oxide Al2O3 is formed.
The high bond strength in Al2О3 determines the high heat of its formation from simple substances and the ability of aluminum to reduce many metals from their oxides, for example:
3Fe3O4 + 8Al \u003d 4Al2O3 + 9Fe and even
3CaO + 2Al \u003d Al2O3 + 3Ca.
This method of producing metals is called aluminothermy.
Being in nature
In terms of prevalence in the earth's crust, aluminum takes the first place among metals and the third place among all elements (after oxygen (O) and silicon (Si)), it accounts for about 8, 8% of the mass of the earth's crust. Aluminum is a huge number of minerals, mainly aluminosilicates, and rocks. Aluminum compounds contain granites, basalts, clays, feldspars, etc. But here is the paradox: with a huge number minerals and rocks containing aluminum, deposits of bauxite - the main raw material in the industrial production of aluminum, are quite rare. In the Russian Federation, bauxite deposits are found in Siberia and the Urals. Alunites and nepheline are also of industrial importance. As a trace element, aluminum is present in the tissues of plants and animals. There are organisms — hubs that accumulate aluminum in their organs — some stalks, mollusks.
Industrial production: at the industrial production index, bauxites are first subjected to chemical processing, removing impurities of silicon oxides (Si), iron (Fe) and other elements from them. As a result of such processing, pure aluminum oxide Al2O3 is obtained - the main one in the production of metal by electrolysis. However, due to the very high melting point of Al2O3 (over 2000 ° C), it is not possible to use its melt for electrolysis.

Scientists and engineers found a way out in the following. The cryolite Na3AlF6 is first melted in the electrolysis bath (the melt temperature is slightly lower than 1000 ° C). Cryolite can be obtained, for example, in the processing of nepheline Kola Peninsula. Then, a little Al2O3 (up to 10% by weight) and some other substances that improve the conditions for subsequent the process. During the electrolysis of this melt, aluminum oxide decomposes, cryolite remains in the melt, and molten aluminum forms at the cathode:
2Al2O3 \u003d 4Al + 3O2.
Aluminum alloys
Most metal elements are alloyed with aluminum, but only some of them play the role of the main alloying components in industrial aluminum alloys. However, a significant number of elements are used as additives to improve the properties of alloys. The most widely used:
Beryllium is added to reduce oxidation at elevated temperatures. Small additives of beryllium (0, 01 - 0, 05%) are used in aluminum cast alloys to improve fluidity in the production of parts of internal combustion engines (pistons and cylinder heads).
Boron is introduced to increase electrical conductivity and as a refining additive. Boron is introduced into aluminum alloys used in nuclear energy (except for reactor parts), because it absorbs neutrons, preventing the spread of radiation. Boron is introduced on average in an amount of 0, 095 - 0, 1%.
Bismuth. Metals with a low melting point, such as bismuth, cadmium, are introduced into aluminum alloys to improve machinability. These elements form soft fusible phases, which contribute to the fragility of the chips and lubrication of the cutter.
Gallium is added in an amount of 0, 01 - 0, 1% to alloys, from which consumable anodes are further made.
Iron. In small quantities (»0.04%) is introduced in the manufacture of wires to increase strength and improves creep characteristics. Same iron reduces sticking to mold walls when casting in a chill mold.
Indium. Additive 0, 05 - 0, 2% harden aluminum alloys during aging, especially with a low cuprum content. Indium additives are used in aluminum-cadmium bearing alloys.
About 0.3% cadmium is introduced to increase strength and improve the corrosion properties of the alloys.
Calcium gives plasticity. With a calcium content of 5%, the alloy has a superplastic effect.
Silicon is the most used additive in cast alloys. In an amount of 0, 5 - 4% reduces the tendency to crack formation. The combination of silicon and magnesium makes it possible to heat seal the alloy.
Magnesium. The addition of magnesium significantly increases strength without reducing ductility, increases weldability and increases the corrosion resistance of the alloy.
Copper hardens alloys, maximum hardening is achieved when the content cuprum 4 - 6%. Cuprum alloys are used in the production of pistons of internal combustion engines, high-quality cast parts of aircraft.
Tin improves cutting performance.
Titanium. The main task of titanium in alloys is grinding grain in castings and ingots, which greatly increases the strength and uniformity of properties in the entire volume.
Although aluminum is considered one of the least noble industrial metals, it is quite stable in many oxidizing environments. The reason for this behavior is the presence of a continuous oxide film on the surface of aluminum, which immediately forms again in the cleaned areas when exposed to oxygen, water and other oxidizing agents.
In most cases, melting is carried out in air. If the interaction with air is limited by the formation on the surface of melt-insoluble compounds and the resulting film of these compounds significantly slows down further interaction, then usually no measures are taken to suppress such interaction. Melting in this case is carried out with direct contact of the melt with the atmosphere. This is what happens in the preparation of most aluminum, zinc, tin - lead alloys.
The space in which the melting of alloys proceeds is limited by a refractory lining capable of withstanding temperatures of 1500 - 1800 ° C. In all melting processes, the gas phase is involved, which is formed in the process of fuel combustion, interacting with the environment and the lining of the melting unit, etc.
Most aluminum alloys have high corrosion resistance in the natural atmosphere, sea water, solutions of many salts and chemicals, and in most food products. Aluminum alloy structures are often used in seawater. Marine buoys, lifeboats, ships, and barges have been built from aluminum alloys since 1930. At present, ship hulls made of aluminum alloys reach a length of 61 m. There is experience in aluminum underground pipelines, aluminum alloys are highly resistant to soil corrosion. In 1951, a pipeline of length 2, 9 km was built in Alaska. After 30 years of operation, no leaks or serious damage due to corrosion were detected.
Aluminum in large quantities is used in construction in the form of cladding panels, doors, window frames, electric cables. Aluminum alloys are not subject to strong corrosion for a long time when in contact with concrete, mortar, plaster, especially if the structures are not subjected to frequent wetting. With frequent wetting, if the surface is aluminum items of trade has not been further processed, it can darken, up to blackening in industrial cities with a high content of oxidizing agents in the air. To avoid this, special alloys are produced to obtain shiny surfaces by brilliant anodizing - applying an oxide film to the metal surface. In this case, the surface can be given many colors and shades. For example, alloys of aluminum with silicon allow you to get a range of shades, from gray to black. Alloys of aluminum with chrome have a gold color.
Industrial aluminum is produced in the form of two types of alloys - cast alloys, parts of which are made by casting, and deformational alloys - alloys produced in the form of deformable semi-finished products - sheets, foils, plates, profiles, wire. Castings from aluminum alloys are obtained by all possible casting methods. Most common under pressure, in chill molds and in sandy-clay forms. In the manufacture of small political parties casting in plaster combined forms and casting Lost wax models. Cast rotors of electric motors, cast parts of aircraft, etc. are made of cast alloys. Deformable alloys are used in automobile manufacturing for interior decoration, bumpers, body panels and interior parts; in construction as a finishing material; in aircraft, etc.
IN industry aluminum powders are also used. Used in metallurgy industry: in aluminothermy, as alloying additives, for the manufacture of semi-finished products by pressing and sintering. This method produces very durable parts (gears, bushings, etc.). Also, powders are used in chemistry to produce aluminum compounds and as catalyst (for example, in the production of ethylene and acetone). Given the high reactivity of aluminum, especially in the form of powder, it is used in explosives and solid fuel for rockets, using its ability to quickly ignite.
Given the high oxidation resistance of aluminum, the powder is used as a pigment in coatings for painting equipment, roofs, printing paper, shiny surfaces of car panels. Also, a layer of aluminum is coated with steel and cast iron subject of trade in order to avoid their corrosion.
In terms of scale of application, aluminum and its alloys take the second place after iron (Fe) and its alloys. The widespread use of aluminum in various fields of technology and everyday life is associated with a combination of its physical, mechanical and chemical properties: low density, corrosion resistance in atmospheric air, high heat and electrical conductivity, ductility and relatively high strength. Aluminum is easily processed in various ways - forging, stamping, rolling, etc. Pure aluminum is used to make wire (the electrical conductivity of aluminum is 65.5% of the electrical conductivity of cuprum, but aluminum is more than three times lighter than cuprum, so aluminum is often replaced in electrical engineering) and foil used as packaging material. The main part of the melted aluminum is spent on obtaining various alloys. Protective and decorative coatings are easily applied to the surface of aluminum alloys.
The variety of properties of aluminum alloys is due to the introduction of various additives into aluminum, forming solid solutions or intermetallic compounds with it. The bulk of aluminum is used to obtain light alloys - duralumin (94% - aluminum, 4% copper (Cu), 0.5% magnesium (Mg), manganese (Mn), (Fe) and silicon (Si)), silumin ( 85-90% - aluminum, 10-14% silicon (Si), 0, 1% sodium (Na)), etc. In metallurgy, aluminum is used not only as a basis for alloys, but also as one of the widely used alloying additives in alloys based on cuprum (Cu), magnesium (Mg), iron (Fe),\u003e nickel (Ni), etc.
Aluminum alloys are widely used in everyday life, in construction and architecture, in the automotive industry, in shipbuilding, aviation and space technology. In particular, the first artificial Earth satellite was made of aluminum alloy. Alloy of aluminum and zirconium (Zr) - is widely used in nuclear reactor engineering. Aluminum is used in the manufacture of explosives.
When handling aluminum in everyday life, you need to keep in mind that only neutral (acidic) liquids (for example, boiling water) can be heated and stored in aluminum containers. If, for example, sour cabbage soup is cooked in aluminum utensils, then aluminum goes into food, and it gets an unpleasant “metallic” taste. Since the oxide film is very easy to damage in everyday life, the use of aluminum utensils is still undesirable.
Silver-white metal, light
density - 2.7 g / cm
the melting temperature of industrial aluminum is 658 ° C, for high-purity aluminum - 660 ° C
specific heat of fusion - 390 kJ / kg
boiling point - 2500 ° C
specific heat of vaporization - 10.53 MJ / kg
temporary resistance of cast aluminum - 10-12 kg / mm2, wrought - 18-25 kg / mm2, alloys - 38-42 kg / mm2
Brinell hardness - 24 ... 32 kgf / mmІ
high ductility: technical - 35%, clean - 50%, rolled into a thin sheet and even foil
Young's modulus - 70 GPa
Aluminum has a high electrical conductivity (0.0265 μOhm · m) and thermal conductivity (203.5 W / (m · K)), 65% of the electrical conductivity of cuprum, has a high reflectivity.
Weak paramagnet.
The linear expansion temperature coefficient is 24.58 · 10−6 K − 1 (20 ... 200 ° C).
The temperature coefficient of electrical resistance is 2.7 · 10−8K − 1.
Aluminum forms alloys with almost all metals. The most famous alloys with cuprum and magnesium (duralumin) and silicon (silumin).
Natural aluminum consists almost entirely of a single stable isotope 27Al with traces of 26Al, a radioactive isotope with period half-life of 720 thousand years, formed in the atmosphere during the bombardment of argon nuclei by protons of cosmic rays.
In terms of prevalence in the earth's crust, the Earth is 1st among metals and 3rd among elements, second only to oxygen and silicon. aluminum content in the earth's crust given various researchers ranged from 7.45 to 8.14% of the mass of the earth's crust.
In nature, aluminum, due to its high chemical activity, occurs almost exclusively in the form of compounds. Some of them:
Bauxites - Al2O3 · H2O (with impurities SiO2, Fe2O3, CaCO3)
Alunites - (Na, K) 2SO4Al2 (SO4) 3Al (OH) 3
Alumina (mixtures of kaolins with sand SiO2, limestone CaCO3, magnesite MgCO3)
Corundum (sapphire, ruby, emery) - Al2O3
Kaolinite - Al2O3 · 2SiO2 · 2H2O
Beryl (emerald, aquamarine) - 3ВеО · Al2О3 · 6SiO2
Chrysoberyl (alexandrite) - BeAl2O4.
However, in some specific reducing conditions, the formation of native aluminum is possible.
In natural waters, aluminum is contained in the form of low-toxic chemical compounds, for example, aluminum fluoride. The type of cation or anion depends, first of all, on the acidity of the aqueous medium. Concentrations of aluminum in surface water bodies Russian Federation range from 0.001 to 10 mg / l, in sea water 0.01 mg / l.

Aluminum (Aluminum) is
Obtaining castings from aluminum alloys
The main challenge facing the foundry in our the country, consists in a substantial overall improvement in the quality of castings, which should find expression in a decrease in wall thickness, a reduction in allowances for machining and for gate-feeding systems while maintaining the proper operational properties of the items sold. The final result of this work should be to provide the increased needs of mechanical engineering with the necessary number of cast billets without a significant increase in the total monetary emission of castings by weight.
Sand casting
Of the above methods of casting in single forms, the most widely used in the manufacture of castings from aluminum alloys has received casting in raw sand forms. This is due to the low density of alloys, the small force of the metal on the mold and low casting temperatures (680-800C).
For the manufacture of sand forms, molding and core mixtures made from quartz and clay sands (GOST 2138–74), molding clays (GOST 3226–76), binders and auxiliary materials are used.

The type of gating system is selected taking into account the dimensions of the casting, the complexity of its configuration and location in the mold. Casting molds for castings of complex configuration of small height is carried out, as a rule, using the lower gate systems. With a high height of castings and thin walls, it is preferable to use vertical slotted or combined gate systems. Molds for small castings are permissible to fill through the upper gating systems. At the same time, the height of the metal scab fall into the mold cavity should not exceed 80 mm.
To reduce the speed of movement of the melt at the entrance to the cavity of the casting mold and better separation of the oxide captures suspended in it and slag inclusions, additional hydraulic resistances are introduced into the gating systems - they install grids (metal or fiberglass) or pour through granular filters.
Sprues (feeders), as a rule, are brought to thin sections (walls) of castings distributed around the perimeter, taking into account the amenities, their subsequent separation during processing. The supply of metal to massive nodes is unacceptable, as it causes the formation of shrinkage shells, increased roughness and shrinkage "dips" on the surface of the castings. In the section, the gate channels are most often rectangular in shape with a wide side of 15–20 mm and a narrow side 5–7 mm in size.
Alloys with a narrow crystallization interval (AL2, AL4, AL), AL34, AK9, AL25, ALZO) are prone to the formation of concentrated shrinkage shells in the heat nodes of castings. The installation of massive profits is widely used to move these shells outside the castings. For thin-walled (4-5 mm) and small castings, the profit mass is 2-3 times greater than the mass of castings, for thick-walled castings - up to 1.5 times. Height arrived choose depending on the height of the casting. At a height of less than 150 mm, height arrived H-arr. take equal to the height of the casting Notl. For higher castings, the Nprib / Notl ratio is taken to be 0, 3 0, 5.
The greatest application in casting aluminum alloys is found in the upper open profits of round or oval cross-section; lateral profits in most cases make closed. To increase work efficiency profits they are insulated, filled with hot metal, topped up. Warming is usually carried out by a sticker on the surface of the sheet asbestos form, followed by drying with a gas flame. Alloys with a wide crystallization interval (AL1, AL7, AL8, AL19, ALZZ) are prone to the formation of diffuse shrinkage porosity. Shrink pore impregnation with profits ineffective. Therefore, in the manufacture of castings from the above alloys, it is not recommended to use the installation of massive profits. To obtain high-quality castings, directional crystallization is carried out, widely using for this purpose the installation of refrigerators made of cast iron and aluminum alloys. The optimal conditions for directional crystallization are created by a vertical slot-gate system. To prevent gas evolution during crystallization and to prevent the formation of gas-shrinkage porosity in thick-walled castings, crystallization under pressure of 0, 4-0, 5 MPa is widely used. For this, casting molds are placed in autoclaves before pouring, filled with metal and crystallized castings under air pressure. For the manufacture of large-sized (up to 2-3 m high) thin-walled castings, a casting method with successively directed solidification is used. The essence of the method is the sequential crystallization of the casting from the bottom up. For this, the casting mold is installed on the table of the hydraulic elevator and metal tubes heated up to 500–700 ° С with a diameter of 12–20 mm are fulfilled, which serve as risers. The tubes are fixedly fixed in the sprue bowl and cover the holes in them with stoppers. After filling the sprue cup with the melt, the stoppers are lifted, and the alloy flows through the tubes into the sprue wells connected to the mold cavity by slotted sprues (feeders). After the melt level in the wells rises 20-30 mm above the lower end of the tubes, the mechanism for lowering the hydraulic table is turned on. The lowering speed is taken such that the filling of the mold is carried out under a flooded level and the hot metal continuously flows into the upper parts of the mold. This provides directional solidification and allows you to get complex castings without shrinkage defects.

Pouring sand forms with metal is carried out from buckets lined with refractory material. Before filling with metal, buckets with a fresh lining are dried and calcined at 780-800 ° C to remove moisture. I maintain the temperature of the melt before pouring at the level of 720–780 ° С. Forms for thin-walled castings are filled with melts heated to 730–750 ° С, and for thick-walled castings to 700–720 ° С.
Gypsum casting
Gypsum casting is used in cases where high demands are placed on castings for accuracy, surface cleanliness and reproduction of the smallest details of the relief. Compared to sand, gypsum molds have higher strength, dimensional accuracy, better withstand high temperatures, and allow castings of complex configuration with a wall thickness of 1, 5 mm to be obtained according to the 5-6th class of accuracy. Molds are made according to wax or metal (brass,) chrome-plated models. Model plates are made of aluminum alloys. To facilitate the removal of models from forms, their surface is covered with a thin layer of kerosene-stearin lubricant.
Small and medium forms for complex thin-walled castings are made from a mixture consisting of 80% gypsum, 20% quartz sand or asbestos and 60-70% of water (by weight of the dry mix). The composition of the mixture for medium and large forms: 30% gypsum, 60% sand, 10% of asbestos, 40-50% of water. To slow down the setting, 1-2% slaked lime is added to the mixture. The required strength of the forms is achieved by hydration of anhydrous or semi-aquatic gypsum. To reduce strength and increase gas permeability, raw gypsum forms are subjected to hydrothermal treatment - they are kept in an autoclave for 6–10 hours under water vapor pressure of 0, 13–0, 14 MPa, and then in air for 24 hours. After this form is subjected to step drying at 350-500 ° C.

A feature of gypsum forms is their low thermal conductivity. This circumstance makes it difficult to obtain dense castings from aluminum alloys with a wide crystallization interval. Therefore, the main task in the development of the gating system for gypsum molds is to prevent the formation of shrinkage shells, loosening, oxide films, hot cracks and underfilling of thin walls. This is achieved by the use of expanding gate systems, providing a low speed of movement of the melts in the mold cavity, directed by solidification of the heat nodes in the direction of profits with the help of refrigerators, by increasing the flexibility of the molds by increasing the content of quartz sand in the mixture. Filling of thin-walled castings is carried out in molds heated to 100-200 ° C by vacuum absorption, which allows filling cavities with a thickness of up to 0.2 mm. Thick-walled (more than 10 mm) castings are obtained by pouring molds in autoclaves. The crystallization of the metal in this case is carried out at a pressure of 0, 4-0, 5 MPa.
Shell casting
Shell casting is expediently used in the serial and large-scale production of castings of limited sizes with increased surface cleanliness, greater dimensional accuracy and less machining than sand casting.
Shell molds are made using hot (250-300 ° С) metal (steel) rigging in a bunker way. Model rigging is performed according to the 4-5th accuracy classes with molding slopes from 0, 5 to 1, 5%. The shells are made two-layer: the first layer from a mixture with 6-10% thermosetting resin, the second from a mixture with 2% resin. For better removal of the shell, the model plate is covered with a thin layer of separation emulsion (5% silicone fluid No. 5; 3% laundry soap; 92% water) before filling the molding sand.
For the manufacture of shell forms, fine-grained quartz sands containing at least 96% silica are used. The connection of the molds is carried out by gluing on special pin presses. Glue composition: 40% resin MF17; 60% marshallite and 1, 5% aluminum chloride (hardening). Filling of the assembled forms is carried out in containers. When casting in shell molds, the same gating systems and temperature conditions are used as when casting in sand forms.
The low rate of crystallization of the metal in shell forms and lower possibilities for creating directional crystallization result in castings with lower properties than when casting in raw sand forms.
Lost wax casting
Lost wax casting is used for the manufacture of castings with increased accuracy (3-5th grade) and surface cleanliness (4th-6th roughness class), for which this method is the only possible or optimal.
Models in most cases are made from pasty paraffinostearin (1: 1) compounds by pressing into metal molds (cast and prefabricated) on stationary or rotary plants. In the manufacture of complex castings with dimensions greater than 200 mm, in order to avoid deformation of the models, substances are added to the composition of the model mass that increase the temperature of their softening (fusion).
A suspension of hydrolyzed ethyl silicate (30–40%) and pulverized silica (70–60%) is used as a refractory coating in the manufacture of ceramic molds. Model blocks are sprinkled with calcined sand 1KO16A or 1K025A. Each coating layer is dried in air for 10-12 hours or in an atmosphere containing ammonia vapors. The necessary strength of the ceramic form is achieved with a shell thickness of 4-6 mm (4-6 layers of refractory coating). To ensure a quiet filling of the mold, expanding sprue systems with metal supply to thick sections and massive nodes are used. Castings are usually fed from a massive riser through thickened runners (feeders). For complex castings, it is allowed to use massive profits to power the upper massive units with their mandatory filling from the riser.

Aluminum (Aluminum) is
Smelting of models from forms is carried out in hot (85-90 ° С) water, acidified with hydrochloric acid (0.5–1 cm3 per liter of water) to prevent saponification of stearin. After melting the models, the ceramic molds are dried at 150–170 ° С for 1–2 h, placed in containers, filled with dry filler, and calcined at 600–700 ° С for 5–8 h. Pouring lead to cold and heated forms. The heating temperature (50-300 ° C) of the molds is determined by the wall thickness of the casting. Filling the molds with metal is carried out in the usual way, as well as using vacuum or centrifugal force. Most aluminum alloys are heated to 720-750 ° C before casting.
Chill casting
Chill casting is the main method for the serial and mass production of castings from aluminum alloys, which allows castings of 4-6th accuracy classes with a surface roughness of Rz \u003d 50-20 and a minimum wall thickness of 3-4 mm to be obtained. When casting in a chill mold, along with defects caused by high speeds of the melt in the mold cavity and non-compliance with the requirements of directional solidification (gas porosity, oxide films, shrinkage loosening), the main types of marriage, castings are underfilling and cracks. Cracks are caused by difficult shrinkage. Especially often, cracks occur in castings from alloys with a wide crystallization interval having a large linear shrinkage (1, 25–1, 35%). Prevention of the formation of these defects is achieved by various technological methods.
In the case of supplying metal to thick sections, it should be provided for feeding the supply site with the installation of the feeding boss (profit). All elements of the gate systems are located on the chill connector. The following ratios of the cross-sectional areas of the sprue channels are recommended: for small castings EFst: EFl: EFpit \u003d 1: 2: 3; for large castings EFst: EFfl: EFpit \u003d 1: 3: 6.
To reduce the rate of melt entry into the mold cavity, curved risers, fiberglass or metal mesh, and granular filters are used. The quality of castings from aluminum alloys depends on the rate of rise of the melt in the mold cavity. This speed should be sufficient to guarantee the filling of thin sections of the castings under conditions of increased heat removal and at the same time not cause underfilling caused by incomplete exit of air and gases through the ventilation ducts and profits, turbulence and flowing of the melt during the transition from narrow sections to wide ones. The rate of rise of metal in the mold cavity during casting into a chill mold is somewhat higher than when casting into sand molds. The minimum allowable lifting speed is calculated by the formulas of A. A. Lebedev and N. M. Galdin (see section 5.1, “Sand Casting”).
To obtain dense castings, directional solidification is created, as in sand casting, by the proper location of the casting in the mold and the regulation of heat removal. As a rule, massive (thick) knots of castings are located in the upper part of the chill mold. This makes it possible to compensate for the reduction in their volume during solidification directly from the profits established above them. The regulation of the intensity of the heat sink in order to create directional solidification is carried out by cooling or warming various sections of the mold. To locally increase the heat sink, inserts from a heat-conducting cuprum are widely used, they provide for an increase in the cooling surface of the chill mold due to fins, local cooling of the chill molds with compressed air or water is carried out. To reduce the heat sink intensity, a paint layer with a thickness of 0, 1-0, 5 mm is applied to the working surface of the chill mold. For this purpose, a paint layer 1-1.5 mm thick is applied to the surface of the sprue channels and profits. Slowing down the cooling of metal in profits can also be achieved through local thickening of the walls of the chill mold, the use of various low heat-conducting coatings and the warming of profits with an asbestos sticker. Coloring the working surface of the chill mold improves the appearance of the castings, helps eliminate gas shells on their surface and increases the durability of the chill molds. Before painting, the chill molds are heated to 100-120 ° C. An excessively high heating temperature is undesirable, as this reduces the solidification speed of the castings and the duration term chill mold service. Heating reduces the temperature difference between the casting and the mold and the expansion of the mold by heating it with the metal of the casting. As a result of this, tensile stresses causing cracks appear in the casting. However, just heating the mold is not enough to eliminate the possibility of cracks. Timely removal of the casting from the mold is necessary. It is necessary to remove the casting from the chill mold earlier than the moment when its temperature is equal to the temperature of the chill mold, and the shrink stress reaches its maximum value. Typically, the casting is removed at the moment when it is so strong that it can be moved without destruction (450-500 ° C). At this point, the gating system has not yet acquired sufficient strength and is destroyed by light impacts. The exposure time of the casting in the mold is determined by the speed of solidification and depends on the temperature of the metal, mold temperature and pouring speed.
To eliminate metal sticking, increase service life and facilitate extraction, metal rods are lubricated during operation. The most common lubricant is a water-graphite suspension (3-5% of graphite).
Parts of chill molds that perform the outlines of castings are made of gray cast iron. The wall thickness of the chill molds is assigned depending on the wall thickness of the castings in accordance with the recommendations of GOST 16237–70. The internal cavity in the castings is performed using metal (steel) and sand rods. Sand rods are used to design complex cavities that cannot be made with metal rods. To facilitate the extraction of castings from chill molds, the outer surfaces of castings should have a casting slope of 30 "to 3 ° towards the connector. The inner surfaces of castings made with metal rods should have a slope of at least 6 °. Sudden transitions from thick to thin sections are not allowed in castings The radii of curvature should be at least 3 mm. Holes with a diameter of more than 8 mm for small castings, 10 mm for medium and 12 mm for large cast rods. The optimum ratio of the depth of the hole to its diameter is 0, 7-1.
Air and gases are removed from the chill mold cavity using ventilation ducts located in the plane of the connector and plugs placed in the walls near the deep cavities.
In modern foundries, chill molds are installed on single-position or multi-position semi-automatic foundry machines in which the closing and opening of the chill mold, the installation and removal of rods, the expulsion and removal of the casting are automated. Automatic control of the temperature of heating the chill mold is also provided. Chill filling on machines is carried out using dispensers.
To improve the filling of the thin cavities of the chill molds and the removal of air and gases released during the destruction of the binders, the molds are evacuated, filled with low pressure or using centrifugal force.
Squeeze casting
Squeeze casting is a type of chill casting. It is intended for the manufacture of large-size castings (2500x1400 mm) of panel type with a wall thickness of 2-3 mm. For this purpose, metal molds are used, which are mounted on specialized casting and squeezing machines with one-sided or two-way rapprochement of the molds. A distinctive feature of this casting method is the forced filling of the mold cavity with a wide melt flow when the half-molds approach each other. In the mold there are no elements of a conventional gate system. Data The method is used to make castings from alloys AL2, AL4, AL9, AL34, having a narrow crystallization interval.
The regulation of the cooling rate of the melt is carried out by applying to the working surface of the cavity forms of heat-insulating coatings of various thicknesses (0.05-1 mm). The overheating of the alloys before pouring should not exceed 15–20 ° С above the liquidus temperature. The duration of rapprochement of the half-forms is 5-3 s.
Low pressure casting
Low pressure casting is another form of chill casting. It has been used in the manufacture of large-sized thin-walled castings from aluminum alloys with a narrow crystallization interval (AL2, AL4, AL9, AL34). As well as during chill casting, the outer surfaces of the castings are made in metal form, and the internal cavities are made in metal or sand cores.
For the manufacture of rods using a mixture consisting of 55% quartz sand 1K016A; 13, 5% bold sand P01; 27% pulverized silica; 0.8% pectin glue; 3, 2% resin M and 0.5% kerosene. Such a mixture does not form a mechanical burnout. Forms are filled with metal by the pressure of compressed, dried air (18–80 kPa) supplied to the surface of the melt in a crucible heated to 720–750 ° С. Under the influence of this pressure, the melt is displaced from the crucible into the metal wire, and from it into the gating system and further into the mold cavity. The advantage of low-pressure casting is the ability to automatically control the rate of metal lift in the mold cavity, which allows thin-walled castings to be obtained better than castings by gravity.
Crystallization of alloys in the form is carried out under a pressure of 10-30 kPa before the formation of a solid metal crust and 50-80 kPa after the formation of a crust.
More dense castings of aluminum alloys are obtained by injection molding under low pressure with back pressure. Filling the mold cavity during backpressure molding is carried out due to the pressure difference in the crucible and in the mold (10-60 kPa). Crystallization of the metal in the mold is carried out at a pressure of 0, 4-0.5 MPa. This prevents the release of hydrogen dissolved in the metal and the formation of gas pores. Increased pressure contributes to better nutrition of massive casting units. Otherwise, backpressure casting technology is no different from low pressure casting technology.
In backpressure casting, the advantages of low pressure casting and crystallization under pressure have been successfully combined.
Injection molding
Die-casting from aluminum alloys AL2, ALZ, AL1, ALO, AL11, AL13, AL22, AL28, AL32, AL34 produce castings of complex accuracy 1-3 configurations with wall thicknesses from 1 mm and above, cast holes with a diameter up to 1, 2 mm, cast external and internal thread with a minimum pitch of 1 mm and a diameter of 6 mm. The surface cleanliness of such castings corresponds to the 5-8th roughness classes. The manufacture of such castings is carried out on machines with cold horizontal or vertical pressing chambers, with a specific pressing pressure of 30–70 MPa. Preference is given to machines with a horizontal pressing chamber.
The dimensions and weight of the castings are limited by the capabilities of injection molding machines: the volume of the pressing chamber, the specific pressing pressure (p) and the locking force (0). The projection area (F) of the casting, the sprue channels and the pressing chamber on the movable plate of the mold should not exceed the values \u200b\u200bdetermined by the formula F \u003d 0, 85 0 / p.
Optimum slopes for external surfaces are 45 °; for internal 1 °. The minimum radius of curvature is 0.5-1 mm. Holes greater than 2.5 mm in diameter are cast. Castings from aluminum alloys, as a rule, are machined only on the landing surfaces. The machining allowance is assigned taking into account the dimensions of the casting and ranges from 0, 3 to 1 mm.
For the manufacture of molds, various materials are used. Parts of molds in contact with liquid metal are made of steel ZX2V8, 4X8B2, 4XB2C, mounting plates and clips of matrices are made of steel 35, 45, 50, pins, bushings and guide columns - made of U8A steel.
The supply of metal to the cavity of the molds is carried out using external and internal gate systems. Feeders bring to the casting areas subjected to machining. Their thickness is assigned depending on the wall thickness of the casting at the place of supply and the given character of filling the mold. This dependence is determined by the ratio of the thickness of the feeder to the thickness of the wall of the casting. Smooth, without turbulence and air entrainment, mold filling takes place if the ratio is close to one. For castings with wall thicknesses up to 2 mm. feeders have a thickness of 0.8 mm; with a wall thickness of 3mm. feeder thickness is 1, 2mm; with a wall thickness of 4-6 mm — 2 mm.
To receive the first portion of the melt, enriched with air inclusions, special washing tanks are located near the mold cavity, the volume of which can reach 20 - 40% of the volume of the casting. The washers are connected to the mold cavity by channels whose thickness is equal to the thickness of the feeders. The removal of air and gas from the cavity of the molds is carried out through special ventilation ducts and gaps between the rods (ejectors) and the mold matrix. Ventilation channels are performed in the plane of the connector on the fixed part of the mold, as well as along the movable rods and ejectors. The depth of the ventilation ducts during casting of aluminum alloys is assumed to be 0.05-0.15 mm, and the width of 10-300 mm in order to improve ventilation, molds of the washer cavity with thin channels (0-0-0.5 mm) is connected to the atmosphere .
The main defects of castings obtained by injection molding are air (gas) subcrustal porosity, caused by air capture at high metal inlet speeds into the mold cavity, and shrinkage porosity (or shells) in thermal units. The formation of these defects is greatly influenced by the parameters of the casting technology, pressing speed, pressing pressure, thermal regime of the mold.
The speed of pressing determines the mode of filling the mold. The higher the pressing speed, the faster the melt moves along the sprue channels, the higher the melt inlet speed into the mold cavity. High pressing speeds contribute to better filling of thin and elongated cavities. However, they are the cause of metal capture of air and the formation of subcortical porosity. When casting aluminum alloys, high pressing speeds are used only in the manufacture of complex thin-walled castings. Pressing pressure exerts a great influence on the quality of castings. As it increases, the density of castings increases.
The magnitude of the pressing pressure is usually limited by the magnitude of the locking force of the machine, which should exceed the pressure exerted by the metal on the movable matrix (pF). Therefore, local prepressing of thick-walled castings, known as the Asigay-process, is of great interest. The low speed of metal inlet into the cavity of the molds through large section feeders and the effective prepressing of crystallizing melt with the help of a double plunger make it possible to obtain dense castings.
The quality of the castings is also significantly affected by the temperature of the alloy and the mold. In the manufacture of thick-walled castings of a simple configuration, the melt is cast at a temperature of 20-30 ° C below the liquidus temperature. Thin-walled castings require the use of a melt superheated above the liquidus temperature by 10-15 ° С. To reduce the value of shrink stresses and prevent the formation of cracks in the castings of the mold, they are heated before casting. The following heating temperatures are recommended:
Casting wall thickness, mm 1–2 2–3 3–5 5–8
Heating temperature
molds, ° С 250—280 200—250 160—200 120—160
Thermal stability is ensured by heating (electric) or cooling (water) molds.
To protect the working surface of the molds from sticking and erosion of the melt, to reduce friction when removing the rods and to facilitate the extraction of castings, the molds are lubricated. For this purpose, use greasy (oil with graphite or aluminum powder) or aqueous (salt solutions, aqueous preparations based on colloidal graphite) lubricants.
Significantly increases the density of castings from aluminum alloys during casting with evacuation of molds. For this, the mold is placed in a sealed casing, in which the necessary vacuum is created. Good results can be obtained using the “oxygen process”. To do this, the air in the cavity of the mold is replaced by oxygen. At high inlet speeds of the metal into the mold cavity, causing the melt to trap oxygen, subcrustal porosity is not formed in the castings, since all the trapped oxygen is spent on the formation of finely dispersed aluminum oxides, which do not significantly affect the mechanical properties of the castings. Such castings can be heat treated.
Depending on the requirements of the technical conditions, castings from aluminum alloys can be subjected to various types of control: X-ray, gamma-defectoscopy or ultrasound to detect internal defects; markup for determining dimensional deviations; fluorescent to detect surface cracks; hydro or pneumatic control to assess tightness. The frequency of the listed types of control is specified by technical conditions or determined by the department of the main metallurgist of the plant. Identified defects, if permitted by the technical conditions, are eliminated by welding or impregnation. Argon-arc welding is used for welding underfilling, shells, friability of cracks. Before welding, the defective place is cut so that the walls of the recesses have a slope of 30 - 42 °. Castings are subjected to local or general heating to 300-350C. Local heating is carried out by an acetylene-oxygen flame, general heating is carried out in chamber furnaces. Welding is carried out with the same alloys of which the castings are made, using a non-consumable tungsten electrode with a diameter of 2-6 mm at expense argon 5-12 l / min. The strength of the welding current is usually 25-40 A per 1 mm of electrode diameter.
Porosity in castings is eliminated by impregnation with bakelite varnish, asphalt varnish, drying oil or liquid glass. Impregnation is carried out in special boilers under pressure of 490-590 kPa with preliminary exposure of castings in a rarefied atmosphere (1, 3-6.5 kPa). The temperature of the impregnating liquid is maintained at 100 ° C. After impregnation, the castings are dried at 65-200 ° C, during which the impregnating liquid hardens, and re-controlled.

Aluminum (Aluminum) is
Aluminum application
Widely used as a structural material. The main advantages of aluminum in this quality are lightness, malleability of stamping, corrosion resistance (in air aluminum is instantly coated with a durable Al2O3 film, which prevents its further oxidation), high thermal conductivity, non-toxicity of its compounds. In particular, these properties have made aluminum extremely popular in the manufacture of cookware, aluminum foil in the food industry and for packaging.



The main disadvantage of aluminum as a structural material is its low strength, therefore, to harden it is usually fused with a small amount of cuprum and magnesium (the alloy is called duralumin).
The electrical conductivity of aluminum is only 1.7 times less than that of cuprum, while aluminum is about 4 times cheaper per kilogram, but due to its 3.3 times lower density, it needs about 2 times less weight to get equal resistance . Therefore, it is widely used in electrical engineering for the manufacture of wires, their shielding, and even in microelectronics in the manufacture of conductors in chips. The lower electrical conductivity of aluminum (37 1 / ohm) compared with cuprum (63 1 / ohm) is compensated by an increase in the cross section of aluminum conductors. The disadvantage of aluminum as an electrotechnical material is the presence of a durable oxide film that makes soldering difficult.
Due to its complex of properties it is widely distributed in thermal equipment.
Aluminum and its alloys retain their strength at extremely low temperatures. Due to this, it is widely used in cryogenic technology.
High reflection coefficient combined with low cost and ease of spraying makes aluminum an ideal material for the manufacture of mirrors.
In the production of building materials as a gas-generating agent.
Corrosion and scale resistance are imparted to steel and other alloys, for example, piston ICE valves, turbine blades, oil recovery towers, heat exchange equipment, and also replace galvanizing.
Aluminum sulfide is used to produce hydrogen sulfide.
Research is underway to develop foamy aluminum as a particularly strong and lightweight material.
As a component of termite, mixtures for aluminothermy
Aluminum is used to reduce rare metals from their oxides or halides.
Aluminum is an important component of many alloys. For example, in aluminum bronzes, the main components are copper and aluminum. In magnesium alloys, aluminum is most often used as an additive. For the manufacture of spirals in electric heaters, fechral (Fe, Cr, Al) is used (along with other alloys).

aluminum coffee "height \u003d" 449 "src \u003d" / pictures / investments / img920791_21_Klassicheskiy_italyanskiy_proizvoditel_kofe_iz_alyuminiya.jpg "title \u003d" (! LANG: 21. Classic Italian aluminum coffee maker" width="376" />!}
When aluminum was very expensive, various jewelry items were made from it. So, Napoleon III ordered aluminum buttons, and Dmitry Ivanovich Mendeleev in 1889 was presented with scales with bowls of gold and aluminum. The fashion for them immediately passed when new technologies (developments) for its production appeared, many times reducing the cost. Now aluminum is sometimes used in the manufacture of jewelry.
![]()
![]()
In Japan, aluminum is used in the manufacture of traditional jewelry, replacing.
Aluminum and its compounds are used as a highly efficient rocket fuel in two-component rocket fuels and as a combustible component in solid rocket fuels. The following aluminum compounds are of most practical interest as rocket fuel:
Powdered aluminum as a fuel in solid rocket fuels. It is also used in the form of powder and suspensions in hydrocarbons.
Aluminum hydride.
Boranate aluminum.
Trimethylaluminum.
Triethylaluminum.
Tripropylaluminum.
Triethylaluminum (usually, together with triethylboron) is also used for chemical ignition (that is, as starting fuel) in rocket engines, as it self-ignites in gaseous oxygen.
It has a slight toxic effect, but many water-soluble inorganic aluminum compounds remain in the dissolved state for a long time and can have a harmful effect on humans and warm-blooded animals through drinking water. The most toxic chlorides, nitrates, acetates, sulfates, etc. For humans, the following doses of aluminum compounds (mg / kg body weight) have a toxic effect when ingested.
aluminum acetate - 0.2-0.4;
aluminum hydroxide - 3.7-7.3;
aluminum alum - 2.9.
It primarily affects the nervous system (accumulates in the nervous tissue, leading to severe disorders of the central nervous system). However, the property of neurotoxicity of aluminum began to be studied from the mid-1960s, since the mechanism of its excretion impedes the accumulation of metal in the human body. Under normal conditions, up to 15 mg of element per day can be excreted in the urine. Accordingly, the greatest negative effect is observed in people with impaired renal excretory function.
According to some biological studies, the intake of aluminum into the human body was considered a factor in the development of Alzheimer's disease, but these studies were later criticized and the conclusion about the relationship of one with the other was refuted.
the chemical characteristics of aluminum are determined by its high affinity for oxygen (in minerals aluminum enters oxygen octahedra and tetrahedra), constant valency (3), weak solubility of most natural compounds. In endogenous processes during the solidification of magma and the formation of igneous rocks, aluminum enters the crystal lattice of feldspars, mica, and other minerals - aluminosilicates. In the biosphere, Aluminum is a weak migrant; it is few in organisms and the hydrosphere. In a humid climate, where decomposing residues of copious vegetation form many organic acids, aluminum migrates in soils and waters in the form of organomineral colloidal compounds; aluminum is adsorbed by colloids and precipitates in the lower part of soils. The connection of aluminum with silicon is partially disrupted and minerals are formed in places in the tropics - aluminum hydroxides Aluminum-boehmite, diasporas, hydrargillite. Most of the aluminum is a part of aluminosilicates - kaolinite, beidellite and other clay minerals. Weak mobility determines the residual accumulation of aluminum in the weathering crust of humid tropics. As a result, eluvial bauxites are formed. In past geological epochs, bauxites also accumulated in lakes and the coastal zone of the seas of tropical regions (for example, sedimentary bauxites of Kazakhstan). In the steppes and deserts, where living matter is scarce, and the waters are neutral and alkaline, aluminum hardly migrates. The most vigorous migration of aluminum is in volcanic regions, where strongly acidic river and groundwater rich in aluminum are observed. In places of displacement of acidic waters with alkaline - marine (in estuaries and others), aluminum precipitates with the formation of bauxite deposits.
Aluminum is part of the tissues of animals and plants; in the organs of mammalian animals, from 10-3 to 10-5% of aluminum (per raw material) was found. Aluminum accumulates in the liver, pancreas and thyroid gland. In plant products, the aluminum content ranges from 4 mg per 1 kg of dry matter (potatoes) to 46 mg (yellow turnip), in products of animal origin - from 4 mg (honey) to 72 mg per 1 kg of dry matter (). In the daily human diet, the aluminum content reaches 35-40 mg. Known organisms are aluminum concentrators, for example, plunders (Lycopodiaceae), containing up to 5.3% aluminum in ash, mollusks (Helix and Lithorina), in which 0.2-0.8% aluminum is in ash. Forming insoluble compounds with phosphates, aluminum disrupts the nutrition of plants (absorption of phosphates by the roots) and animals (absorption of phosphates in the intestines).
The main acquirer is aviation. The most heavily loaded elements of the aircraft (casing, power reinforcing set) are made of duralumin. And in space this alloy was taken. And even on the moon he came and returned to Earth. And the stations “Luna”, “Venus”, “Mars”, created by the designers of the bureau, which for many years was headed by George Nikolaevich Babakin (1914-1971), could not do without aluminum alloys.
Alloys of the aluminum - manganese and aluminum - magnesium systems (AMts and AMg) are the main material of the hulls of high-speed "rockets" and "meteors" - hydrofoils.
But not only in space, aviation, sea and river transport, aluminum alloys are used. Aluminum holds a strong position in ground transport. The widespread use of aluminum in the automotive industry is indicated by such data. In 1948, 3.2 kg of aluminum was used per one, in 1958 - 23.6, in 1968 - 71.4, and today this figure exceeds 100 kg. Appeared aluminum and rail. And the Russian Express super express is more than 50% made of aluminum alloys.
Aluminum is increasingly being used in construction. In new buildings, strong and light beams, floors, columns, railings, fences, elements of ventilation systems made of aluminum-based alloys are often used. In recent years, aluminum alloys have entered the construction of many public buildings, sports complexes. There are attempts to use aluminum as a roofing material. Such a roof is not afraid of carbon dioxide admixtures of sulfur compounds, nitrogen compounds and other harmful impurities, which extremely intensify the atmospheric corrosion of roofing iron.
As casting alloys, silumins are used - alloys of the aluminum - silicon system. Such alloys have good fluidity, give small shrinkage and segregation (heterogeneity) in the castings, which allows casting the most complicated parts by molding method, for example, engine housings, pump impellers, instrument housings, internal combustion engine blocks, pistons, heads and cylinder shirts piston engines.
Fight for decline cost aluminum alloys have also been crowned with success. For example, silumin is 2 times cheaper than aluminum. Usually, on the contrary, alloys are more expensive (to get an alloy, you need to get a clean base, and then alloying to get an alloy). Soviet metallurgists at the Dnepropetrovsk aluminum plant in 1976 mastered the production of silumins directly from aluminosilicates.
Aluminum has long been known in electrical engineering. However, until recently, the scope of aluminum was limited to power lines and, in rare cases, power cables. The cable industry was dominated by copper and lead. The conductive elements of the cable construction were made of cuprum, and the metal sheath was made of lead or lead based alloys. For many decades (for the first time lead sheaths for protecting cable cores were proposed in 1851) was the only metal material for cable sheaths. He is excellent in this role, but not without flaws - high density, low strength and scarcity; these are only the main ones that made a person look for other metals that can adequately replace lead.
It turned out to be aluminum. The beginning of his service in this role can be considered 1939, and work was begun in 1928. However, a serious shift in the use of aluminum in cable technology occurred in 1948, when the technology for manufacturing aluminum shells was developed and mastered.
Copper, too, for many decades was the only metal for the manufacture of live conductors. Studies of materials that could replace copper have shown that aluminum should and can be such a metal. So, instead of two metals, essentially of various purposes, aluminum entered cable technology.
Such a replacement has several advantages. Firstly, the possibility of using an aluminum sheath as a zero conductor is a significant metal saving and weight reduction. Secondly, higher strength. Thirdly, - easier installation, reduced transportation costs, reduced cable costs, etc.
Aluminum wires are also used for overhead power lines. But it took a lot of effort, time to make an equivalent replacement. Many options have been developed, and they are used based on the specific situation. [Aluminum wires of increased strength and increased creep resistance are manufactured, which is achieved by alloying with magnesium up to 0.5%, silicon up to 0.5%, iron up to 0.45%, hardening and aging. Steel-aluminum wires are used, especially for the performance of large spans required at the places where power lines cross various obstacles. There are spans of more than 1,500 m, for example, when crossing rivers.
Transmission Aluminum electricity long distances are used not only as a conductive material. A dozen and a half years ago, aluminum-based alloys began to be used for the manufacture of transmission line poles. They were first built in our the country in the Caucasus. They are lighter than steel by about 2.5 times and do not require corrosion protection. Thus, the same metal displaced iron, copper and lead in electrical engineering and the technique of transmitting electricity.
And so or almost so was in other areas of technology. In the oil, gas and chemical industries, tanks, pipelines and other assembly units made of aluminum alloys have proven themselves well. They supplanted many corrosion-resistant metals and materials, such as containers made of iron-carbon alloys, enameled inside to store aggressive liquids (a crack in the enamel layer of this expensive construction could lead to losses or even an accident).
Over 1 million tons of aluminum is consumed annually in the world for the production of foil. The thickness of the foil, depending on its purpose, is in the range of 0.004-0.15 mm. Its application is extremely diverse. It is used for packaging various food and industrial products - chocolate, sweets, medicines, cosmetics, photo products, etc.
Foil is also used as a structural material. There is a group of gas-filled plastics - honeycomb plastics - cellular materials with a system of regularly repeating cells of regular geometric shapes, the walls of which are made of aluminum foil.


Encyclopedia of Brockhaus and Efron
WHAT IS ALUMINUM
Lightweight, durable, resistant to corrosion and functional - this combination of qualities has made aluminum the main structural material of our time. Aluminum is in the houses in which we live, cars, trains, and airplanes, where we travel distances, in mobile phones and computers, on refrigerator shelves, and in modern interiors. But 200 years ago, little was known about this metal.
“What seemed unrealizable for centuries, which yesterday was only a bold dream, today is becoming a real challenge, and tomorrow is an accomplishment.”
Sergey Pavlovich Korolev
scientist, designer, founder of practical cosmonautics
Aluminum - silver-white metal, the 13th element of the periodic table. It is incredible, but true: aluminum is the most abundant metal on Earth, it accounts for more than 8% of the total mass of the earth's crust, and this is the third most abundant chemical element on our planet after oxygen and silicon.
At the same time, aluminum does not occur in nature in its pure form due to its high chemical activity. That is why we learned about it relatively recently. Formally, aluminum was obtained only in 1824, and another half century passed before its industrial production began.
Most often in nature, aluminum is found in alum. These are minerals that combine two salts of sulfuric acid: one based on an alkali metal (lithium, sodium, potassium, rubidium or cesium), and the other based on the metal of the third group of the periodic table, mainly aluminum.
Alum is used today in water purification, in cooking, medicine, cosmetology, in chemical and other industries. By the way, aluminum got its name precisely thanks to alum, which in Latin was called alumen.

Corundum
Rubies, sapphires, emeralds and aquamarine are aluminum minerals.
The first two relate to corundum - this is alumina (Al 2 O 3) in crystalline form. It has natural transparency, and in strength is second only to diamonds. Bulletproof glass, portholes on airplanes, smartphone screens are made using sapphire.
And one of the less valuable corundum minerals - emery is used as an abrasive material, including for creating sandpaper.
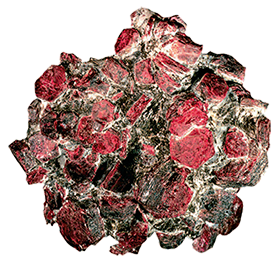
Today, almost 300 different compounds and minerals of aluminum are known - from feldspar, which is the main rock-forming mineral on Earth, to ruby, sapphire or emerald, which are not so common.

Hans Christian Oersted (1777–1851) - Danish physicist, honorary member of the St. Petersburg Academy of Sciences (1830). Born in the city of Rudkörbing in the family of a pharmacist. In 1797 he graduated from the University of Copenhagen, in 1806 - became a professor.
But no matter how common aluminum was, its discovery became possible only when a new tool appeared at the disposal of scientists, which allows splitting complex substances into simple ones, - electricity.
And in 1824, using the electrolysis process, Danish physicist Hans Christian Oersted obtained aluminum. It was contaminated with impurities of potassium and mercury involved in chemical reactions, but this was the first case of aluminum production.
Using electrolysis, aluminum is produced today.
The raw material for the production of aluminum today is another aluminum ore common in nature - bauxite. This is a clay rock, consisting of various modifications of aluminum hydroxide mixed with oxides of iron, silicon, titanium, sulfur, gallium, chromium, vanadium, carbonate salts of calcium, iron and magnesium - almost half of the periodic table. On average, 1 ton of aluminum is produced from 4-5 tons of bauxite.
Bauxites
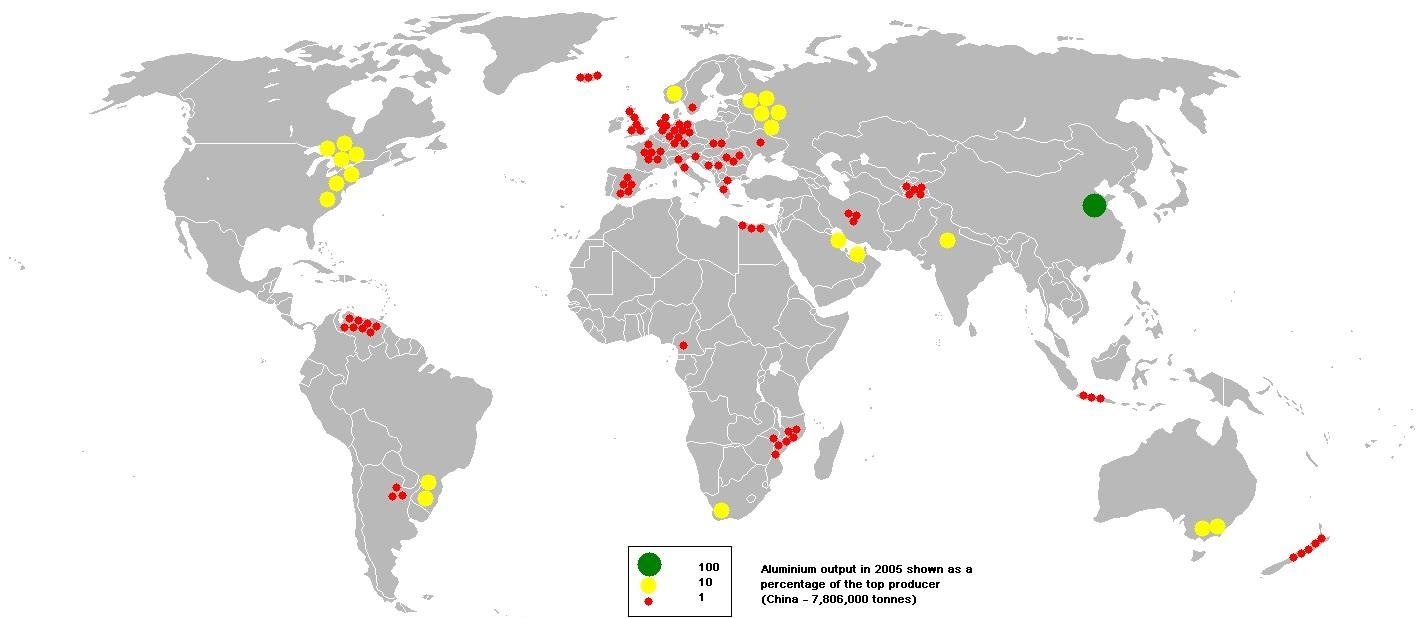
Bauxites in 1821 were discovered by geologist Pierre Bertier in southern France. The breed got its name in honor of the locality of Les Baux, where it was found. About 90% of the world's bauxite reserves are concentrated in the countries of the tropical and subtropical zones - in Guinea, Australia, Vietnam, Brazil, India and Jamaica.

From bauxite get alumina. This is aluminum oxide Al 2 O 3, which has the form of a white powder and from which metal is produced by electrolysis in aluminum plants.
Aluminum production requires a huge amount of electricity. To produce one ton of metal, about 15 MWh of energy is needed - so much a 100-apartment building consumes for a whole month. Therefore, it is most reasonable to build aluminum plants near powerful and renewable energy sources. The best solution is hydro power plantsrepresenting the most powerful of all types of "green energy".
Aluminum properties
Aluminum has a rare combination of valuable properties. This is one of the lightest metals in nature: it is almost three times lighter than iron, but it is strong, extremely ductile and not subject to corrosion, since its surface is always covered with the thinnest, but very strong oxide film. It does not magnetize, perfectly conducts electric current and forms alloys with almost all metals.

Easy
Three times lighter than iron

Lasting
Comparable in strength to steel

Plastic
Amenable to all types of machining

No corrosion
Thin oxide film protects against corrosion
Aluminum is easily processed by pressure, both in hot and in cold condition. It lends itself to rolling, drawing, stamping. Aluminum does not burn, does not require special coloring and is not toxic unlike plastic.
The ductility of aluminum is very high: from it it is possible to make sheets with a thickness of only 4 microns and the thinnest wire. And ultra-thin aluminum foil is three times thinner than a human hair. In addition, compared to other metals and materials, it is more economical.
The high ability to form compounds with various chemical elements gave rise to many aluminum alloys. Even a small fraction of impurities significantly changes the characteristics of the metal and opens up new areas for its application. For example, the combination of aluminum with silicon and magnesium in everyday life can be found literally on the road - in the form of alloy wheels, engines, in chassis elements and other parts of a modern car. And if you add zinc to the aluminum alloy, then perhaps you are now holding it in your hands, because it is this alloy that is used in the manufacture of cases for mobile phones and tablets. Meanwhile, scientists continue to invent new and new aluminum alloys.
Aluminum reserves
About 75% of the aluminum produced over the entire life of the industry is still in use.
The article used photographs © Shutterstock and © Rusal.
Lesson type. Combined.
Tasks:
Educational:
1. Actualize students' knowledge about the structure of the atom, the physical senses of the serial number, group number, period number using the example of aluminum.
2. To form knowledge among students that aluminum in a free state has special, characteristic physical and chemical properties.
Developing:
1. To arouse interest in the study of science by providing brief historical and scientific reports on the past, present and future of aluminum.
2. To continue the formation of students' research skills when working with literature and laboratory work.
3. To expand the concept of amphoteric disclosure of the electronic structure of aluminum, the chemical properties of its compounds.
Educational:
1. To foster a respect for the environment by providing information about the possible use of aluminum yesterday, today, tomorrow.
2. To form the skills of working as a team for each student, to take into account the opinion of the whole group and to defend their own correctly, performing laboratory work.
3. To acquaint students with the scientific ethics, honesty and decency of natural scientists of the past, providing information about the struggle for the right to be the discoverer of aluminum.
REPETITION OF THE MATERIAL PASSED by topics alkaline and alkaline earth M (REPETITION):
What is the number of electrons at the external energy level of alkaline and alkaline earth M?
What products are formed when interacting with sodium or potassium oxygen? (peroxide), is lithium capable of producing peroxide in reaction with oxygen? (no, lithium oxide is formed as a result of the reaction.)
How to get sodium and potassium oxides? (calcination of peroxides with the corresponding Me, Pr: 2Na + Na 2 O 2 \u003d 2Na 2 O).
Do alkali and alkaline earth metals exhibit negative oxidation states? (No, they do not, because they are strong reducing agents.).
How does the radius of an atom change in the main subgroups (from top to bottom) of the periodic system? (increasing), what is this connected with? (with an increase in the number of energy levels).
Which of the metal groups we have studied are lighter than water? (in alkaline).
Under what conditions is the formation of hydrides in alkaline earth metals? (at high temperatures).
Which substance calcium or magnesium reacts more actively with water? (Calcium reacts more actively. Magnesium reacts actively with water only when it is heated to 100 0 С).
How does the solubility of alkaline earth metal hydroxides in water vary, from calcium to barium? (solubility in water increases).
Tell us about the storage features of alkali and alkaline earth metals, why are they stored in this way? (since these metals are very reactive, they are stored in containers under a layer of kerosene).
CONTROL WORK on the topics of alkaline and alkaline earth M:
LESSON OUTLINE (STUDY OF NEW MATERIAL):
Teacher: Hello guys, today we are going to study the IIIA subgroup. List the elements located in subgroup IIIA?
Trainees: It includes elements such as boron, aluminum, gallium, indium and thallium.
Teacher: What number of electrons do they contain at the external energy level, oxidation state?
Trainees: Three electrons, oxidation state +3, although oxidation state +1 is more stable for thallium.
Teacher: The metallic properties of the elements of the boron subgroup are much less pronounced than those of the elements of the beryllium subgroup. Bohr is not M. Subsequently, within the subgroup, with an increase in the nuclear charge M, the properties are enhanced. Al - already M, but not typical. Its hydroxide has amphoteric properties.
Of the M main subgroups of group III, aluminum is of greatest importance, the properties of which we will study in detail. It is interesting to us because it is a transitional element.


